White House agrees to reopen Abbott Laboratories’ Michigan facility following recall of contaminated baby formulas
05/18/2022 / By Mary Villareal

The White House recently reached an agreement with Abbott Laboratories to reopen its Michigan infant formula production facility. It also said the Food and Drug Administration will work to expedite imports of infant formula products.
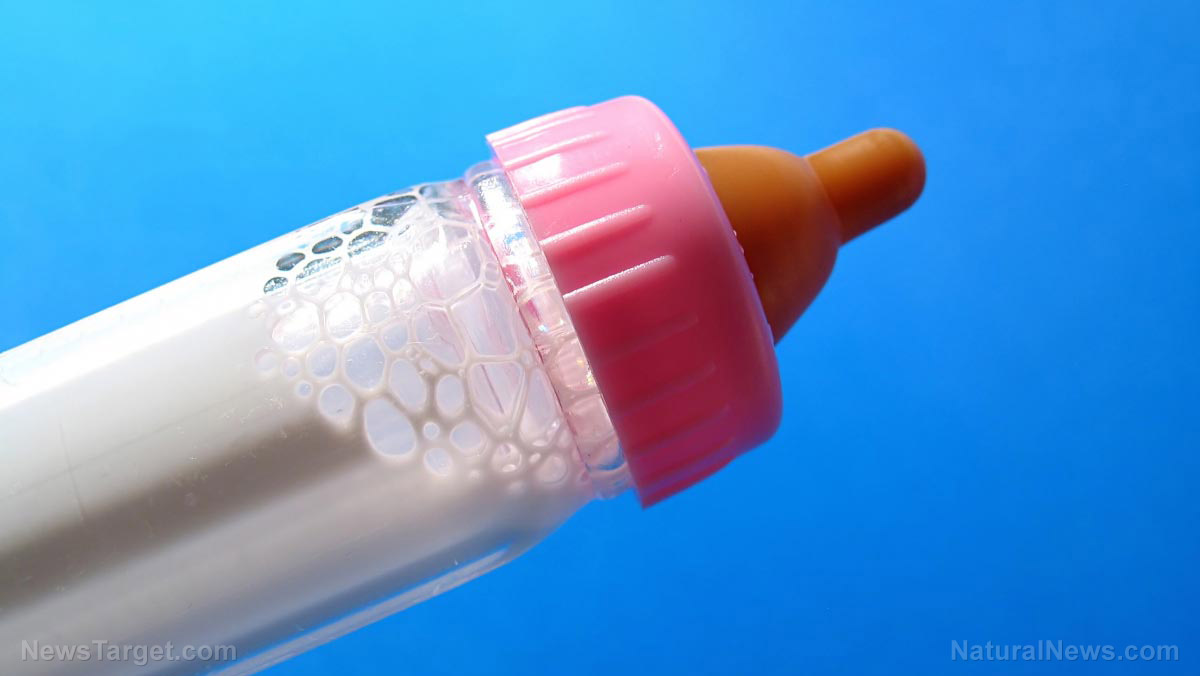
This development comes amid a nationwide shortage attributed to the February shutdown of Abbott Laboratories’ Michigan plant following a voluntary product recall as parents complained of salmonella poisoning in infants who have consumed formula manufactured in the facility.
The White House also announced that the Department of Health and Human Services created a new tool for parents that could help them locate baby formula products. However, the site left parents with long waits and more questions than answers.
Whether or not the announcements will lead to better results, the nationwide shortage has exposed multiple issues related to the infant formula industry, including a near-monopoly on production in the U.S. as well as the restriction of imports from other countries.
The recall of one of the industry’s largest manufacturers, ongoing supply chain issues and a market that is dominated by only a few players have created a “perfect storm” that affected the supply of essential formula to millions of babies across the United States.
Forbes predicted that the infant formula shortage could last for months, although the announcement from the Biden administration suggests that plans are underway to address it sooner rather than later. (Related: Abbott Nutrition’s infant formula was recalled after claims of bacterial contamination, infant deaths.)
So far, there are only three companies that dominate the infant formula market: Abbot, Mead Johnson and Gerber. Market analysis by the U.S. Department of Agriculture (USDA) also showed that these companies accounted for nearly all U.S. formula sales.
The government, under the USDA’s Special Supplemental Nutrition Program for Women, Infants and Children (WIC), is also said to be the largest U.S. purchaser of instant formula. WIC, which provides services to pregnant women, young mothers and their children, awards contracts for infant formula to a handful of approved companies.
Abbott Nutrition, which is the food-sector branch of Abbott Laboratories, dominates the market and accounts for roughly 43 percent of the formula market in 2011. Abbott shut down its formula manufacturing plant in Sturgis, Michigan, and recalled all formula when several infants were reported to become ill from bacterial infection. Two died after consuming formula produced in the plant.
Health and safety issues not new for Abbott’s Michigan plant
Health and safety compliance issues are not new for Abbott’s Michigan plant. In October 2021, a whistleblower submitted a report to the FDA that Abbott falsified records, released untested infant formula, undermined an FDA audit in 2019, had lax cleaning processes and failed to adequately trace its products.
However, it wasn’t until early 2022 that the FDA conducted a formal inspection of the company’s facilities. After the February shutdown and recall, Abbott also conducted its own investigation and concluded that nothing on the facility grounds matched the particular strain of bacteria that caused the illnesses and deaths of the two infants.
The FDA and the Centers for Disease Control and Prevention echoed Abbott’s findings, although the FDA is still continuing its investigation of the plant.
According to the FDA, it would allow the company to release formula from the Michigan plant on a case-by-case basis. However, the agency has been widely criticized for its slowness in responding to and mitigating the crisis.
The White House announcement signals that the FDA will prioritize the review and approval of imports as part of Biden’s strategy to address the shortage.
“The FDA expects that the measures and steps it’s taking with infant formula manufacturers and others will mean more and more supply is on the way or on store shelves moving forward,” said FDA Commissioner Robert Califf.
He added that the U.S. will prioritize companies that can provide the largest shipments and quickly show documentation that their formulas are safe and compatible with U.S. nutrition standards.
This import policy is structured to be temporary and will last for only six months.
Getting imports into the U.S. supply chain will take several weeks, and products from Australia, New Zealand and the U.K. are expected to meet the standards needed for importation.
Meanwhile, fixing the violations uncovered at Abbott’s plant will take time. According to the FDA, the company needs to exhaustively clean its facility and equipment, retrain staff and repeatedly test and document that there is no contamination. (Related: It’ll be at least another 10 weeks before Abbott reopens and starts getting baby formula back in stores.)
Get more info about the baby formula shortage at Products.news.
Watch the video below to know what Americans think about the ongoing baby formula shortage.
This video is from the InfoWars channel on Brighteon.com.
More related stories:
Why is the media reluctant to cover the American baby formula shortage?
Biden sends baby formula to illegals at border while grocery stores run dry.
Sources include:
Submit a correction >>
Tagged Under:
Abbott, baby food, baby formula, baby formula shortage, big government, clean food watch, FDA, food collapse, food supply, grocery, hunger, infant's health, Product recall, products, rationing, Salmonella poisoning, scarcity, starvation, supply chain, supply chain issues, White House
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 WHITE HOUSE NEWS